We are opening a new page on this website dedicated to those who like to build things. On this page we will publish materials sent to us with permission to publish, describing projects which utilize devices manufactured, distributed, sold, donates, loaned by Green Energy Research, Inc, to the author or his organization.
If you would like to submit a project for publication, please contact info@greenenergyresearch.com .
| Project Name: CHEMCAR |
| Author Name and Contact: Sean Burgess seanburgess247@gmail.com |
| ABSTARCT: Project Background
• Unknown distance, between 50-100 ft • Unknown volume of water, between 0-500 mL • Both announced an hour before competition start • Closest to the finish line (over or under) wins |
Car Background
• Powers a proton exchange membrane (PEM) fuel cell • Pressure regulated to fuel cell through pressure regulator • Iodine Clock Reaction (ICR) used as stopping mechanism • Power from fuel cell passed through a circuit • Regulates voltage to the motor • Integrates the ICR stopping mechanism |
| Project Text, Pictures and Links: |
Chemical and Molecular Engineering Car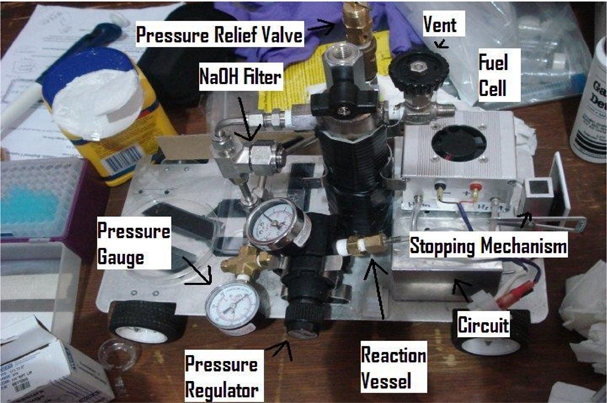 |
Circuit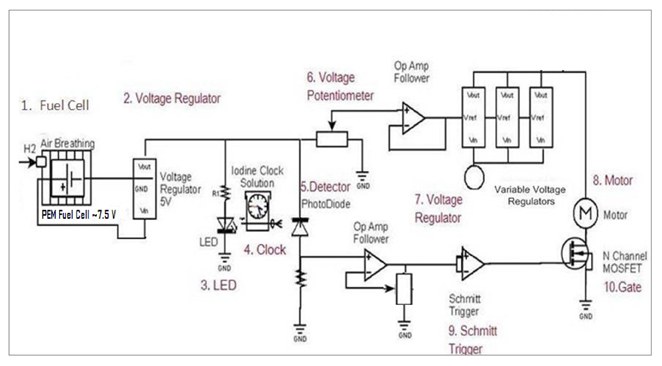 |
| Hydrogen Generation • Reaction vessel is a 1- 1/4" stainless steel pipe • 6.2" high (15.8 cm) • Welded at top and bottom • Rated to 230 PSIG • Powdered aluminum (300 mesh 99.9% )  |
| Hydrogen Generation Results • Overall Reaction: 2Al + 3H2O → Al2O3 + 3H2 • Molecular weight of aluminum: 26.98 g/mol • 0.77g Aluminum (maximum amount of the limiting reagent) = 0.0283 mol of Al • Maximum operating pressure calulation 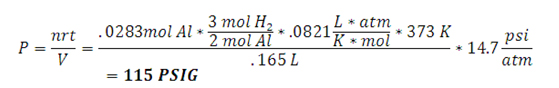 • 40 mL of 1M NaOH is added to 1.2 g Al • To reach completion reaction takes about 20 minutes • 1.2 g Al allows the max pressure to be reached in about 5 minutes • Volume of the system is 0.165 L • Max temperature reached is 373 K • Generation rate of 25 PSI/min 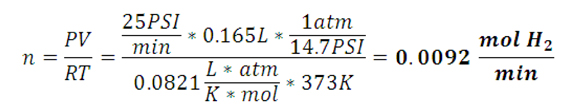 |
| Stopping Mechanism (ICR) The reactions:
• Step 2: (Fast) 2S2O32- + I2 2I- + S4O62-
• Starch indicator gives solution dark blue color
|
Iodine Clock Reaction |
Stopping Mechanism Rate Law contd. Rate determined below
• Found from literature1 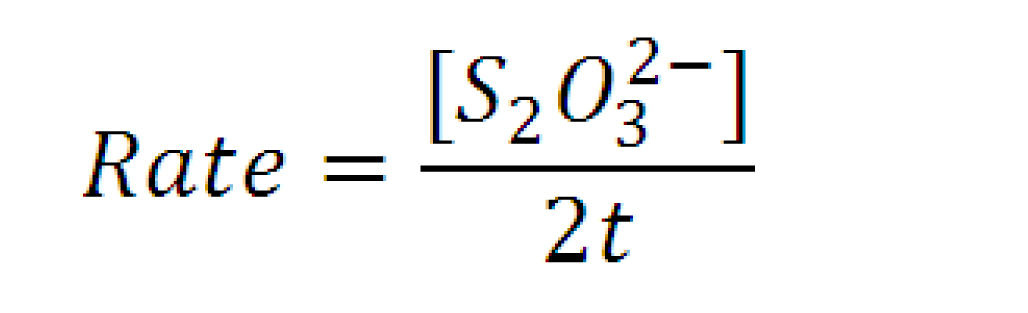 Value for m (slope of Graph A)is 1.5 Value for n (slope of Graph B) is 2.0 [1]. King, Cecil V., and Otto F. Steinbach. "KINETICS OF THE REACTION BETWEEN PERSULFATE AND THIOSULFATE IONS IN DILUTE AQUEOUS SOLUTION." J. Am. Chem. 12th ser. 1930.52 (2002): 4779-795. JSTOR. Web. 9 Apr. 2009. Values m and n extracted from graph
• K=0.31±0.04  Rate law in practice
• [S2O32-] kept constant at 0.0465 M • Volume change made up by water • [I-] determined from Time vs. [I-] graph • [S2O82-] determined from equation below  |
| Speed of the Car Trials at different volumes of water
• Weight plugged in • Multiplied by distance/100 |








